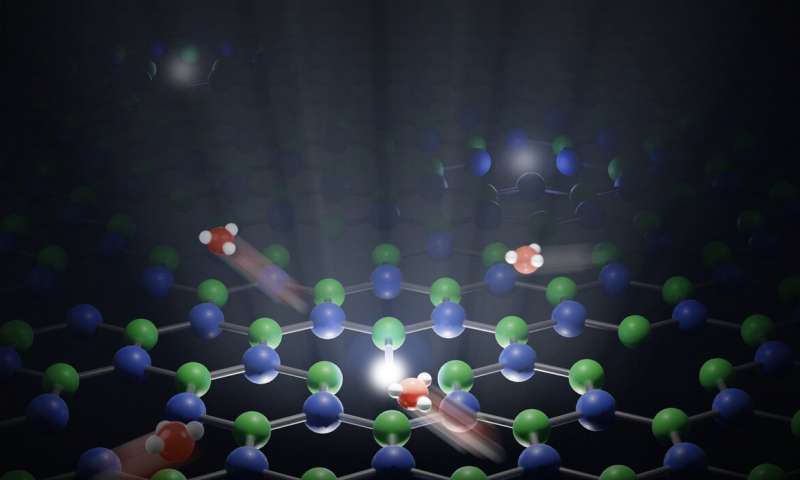
The H+ proton consists of a single ion of hydrogen,
the smallest and lightest of all the chemical elements. These
protons occur naturally in water where a tiny proportion of H2O
molecules separate spontaneously. Their amount in a liquid
determines whether the solution is acidic or basic. Protons are
also extremely mobile, moving through water by jumping from one
water molecule to another.