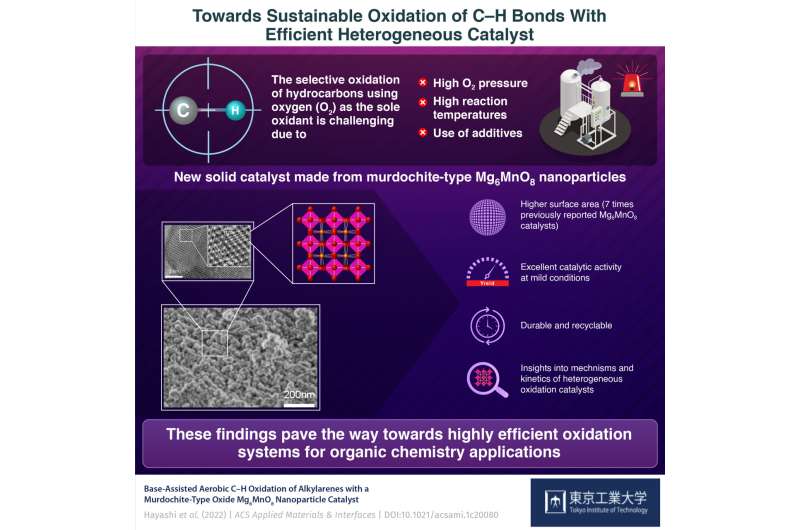
In the chemical industry, the selective cleavage and
oxidation of carbon–hydrogen (C–H) bonds, called “oxidative C–H
functionalization” is an essential step in the production of many
solvents, polymers, and surfactants, as well as intermediate
compounds for agrochemicals and pharmaceuticals. Ideally, one would
want to use oxygen (O2) as the only oxidant in this process to
avoid using more expensive and environmentally taxing substances,
such as hydrogen peroxide (H2O2), chlorine (Cl2), or nitric acid
(HNO3).
Reusable catalyst makes C–H bond oxidation using oxygen
easier and more efficient