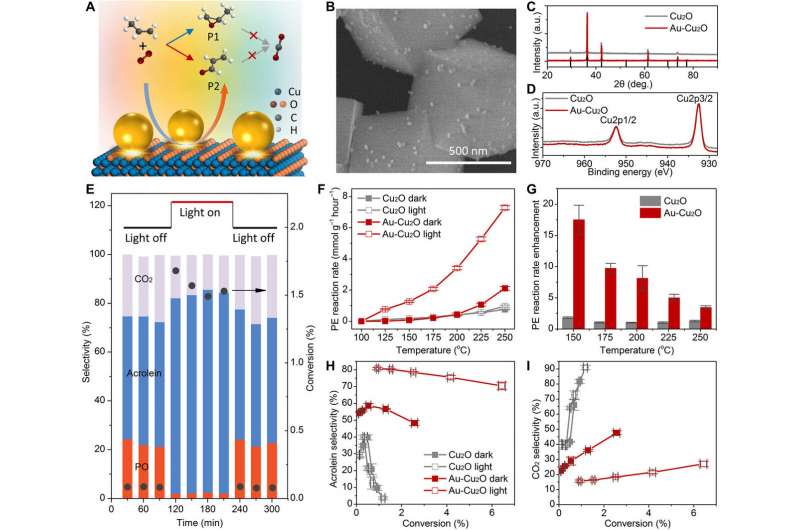
When optimizing catalysis in the lab, product
selectivity and conversion efficiency are primary goals for
materials scientists. Efficiency and selectivity are often mutually
antagonistic, where high selectivity is accompanied by low
efficiency and vice versa. Increasing the temperature can also
change the reaction pathway. In a new report, Chao Zhan and a team
of scientists in chemistry and chemical engineering at the Xiamen
University in China and the University of California, Santa
Barbara, U.S., constructed hierarchical plasmonic nanoreactors to
show nonconfined thermal fields and electrons. The combined
attributes uniquely coexisted in plasmonic nanostructures. The team
regulated parallel reaction pathways for propylene partial
oxidation and selectively produced acrolein during the experiments
to form products that are different from thermal catalysis. The
work described a strategy to optimize chemical processes and
achieve high yields with high selectivity at lower temperature
under visible light illumination. The work is now published on
Science Advances.
Plasmonic nanoreactors regulate selective oxidation via
energetic electrons and nanoconfined thermal fields