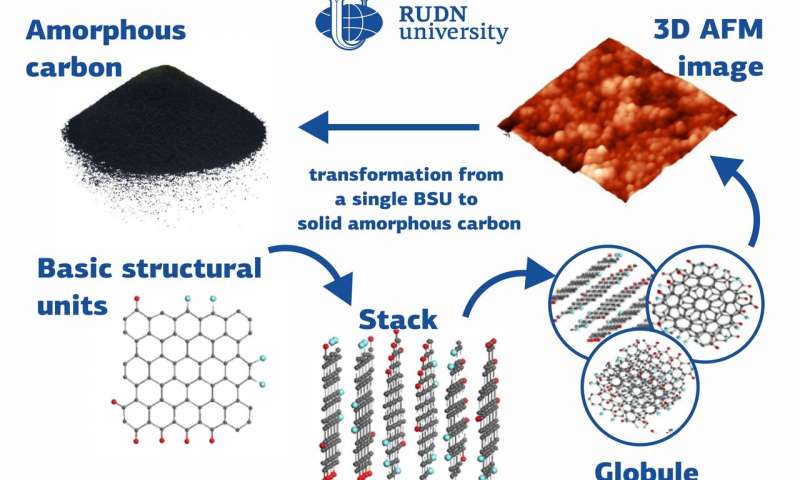
Many substances with different chemical and physical
properties, from diamonds to graphite, are made up of carbon atoms.
Amorphous forms of solid carbon do not have a fixed crystal
structure and consist of structural units—nanosized graphene
particles. A team of physicists from RUDN University studied the
structure of amorphous carbon and suggested classifying it as a
separate type of amorphous solid bodies: a molecular amorphic with
enforced fragmentation. The results of the study were published in
the Fullerenes, Nanotubes and Carbon Nanostructures
journal.