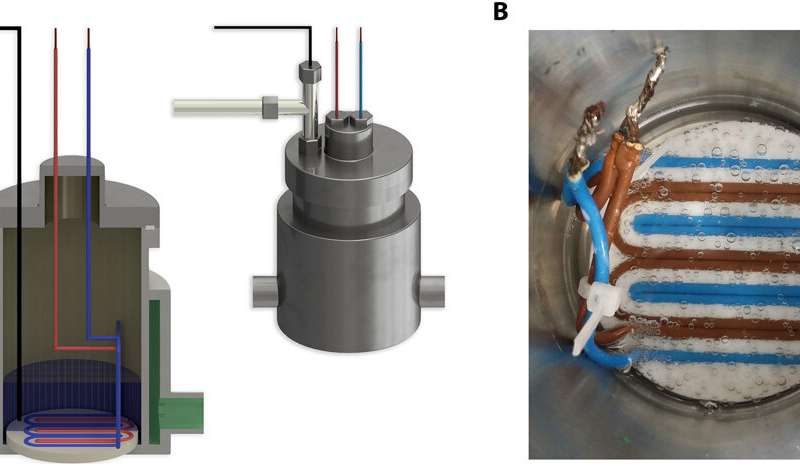
Nanobubbles can exist on solid surfaces or in bulk
liquids as nanoscopic gaseous domains. The phenomenon has attracted
substantial attention due to the long-time (meta)stability and
potential for practical applications. In a new report, Mohammad
Reza Ghaani and a team of researchers in chemistry and bioprocess
engineering in Ireland and Canada used a novel approach to explore
the surface of electrostatic nanobubble (NB) formation. They
observed the stability of the constructs by applying external
electric fields in gas-liquid systems to observe massive gas uptake
into the liquid in nanobubble form. During a period of time lasting
months, the gas solubility enhanced from 2.5 fold for oxygen to
30-fold for methane, based on Henry’s Law values for gas
solubility—i.e., the more hydrophobic the gas, the greater the
intake. Using molecular dynamics solutions, Ghaani et al. revealed
the origin of NB’s movement to result from dielectrophoresis, while
the substantial stability of NB arose from surface-polarization
interactions. The work is now published on Science
Advances.
Massive generation of metastable bulk nanobubbles in water
by external electric fields